Obese and Overweight Patients
Obesity is a common comorbidity of type 2 diabetes. Modest and sustained weight loss has been shown to improve glycemic control and reduce the need for glucose-lowering medications in patients with type 2 diabetes who are overweight or obese. Clinical benefits are usually achieved at 3%-5% weight loss, with even greater benefits observed with progressive weight loss.1
Patients who are overweight or obese should be encouraged to make lifestyle changes, including diet, physical activity, and behavioral changes, to lose weight. Lifestyle programs that achieve a 500-750 kcal/day energy deficit through diet and exercise can lead to clinically significant weight loss.1
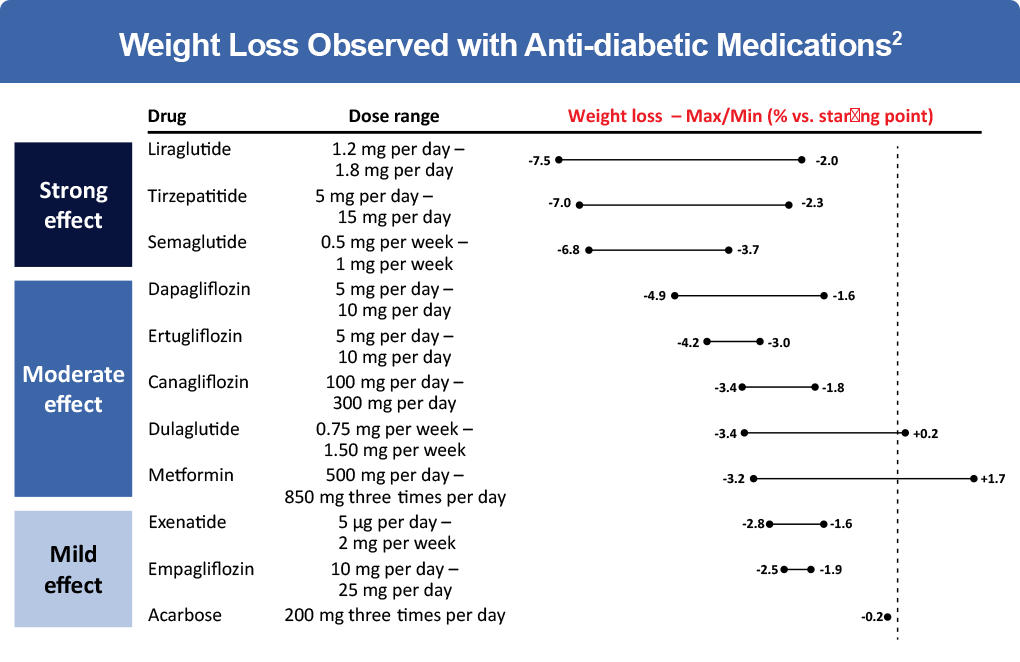
However, weight loss due to lifestyle changes alone is often short-term.2 When selecting therapy for patients with type 2 diabetes who are overweight or obese, medications that reduce weight should be considered.1 New anti-diabetic drugs, particularly GLP-1 receptor agonists and tirzepatide, are the most effective in inducing weight loss in patients with type 2 diabetes.2
Mechanisms of Action of Anti-diabetic Medications that Promote Significant Weight Loss
Among glucose-lowering medications, GLP-1 receptor agonists are associated with the greatest weight loss in patients with type 2 diabetes and several of these agents are FDA approved specifically for weight loss. GLP-1 is an incretin hormone released from the intestinal mucosa in response to the ingestion of food, along with a second incretin hormone, glucose-dependent insulinotropic polypeptide (GIP).3,4 In healthy individuals, GLP-1 and GIP bind to pancreatic beta cells to enhance glucose-stimulated insulin secretion and lower blood glucose levels.4 However, incretin hormones are only able to stimulate insulin release when blood glucose levels are elevated and therefore, are unlikely to induce hypoglycemia.5-7
GLP-1 and GIP are responsible for the “incretin effect,” a phenomenon in which oral glucose leads to a significantly greater insulin response than intravenous glucose, even when the same plasma glucose concentrations are achieved.3 The incretin effect accounts for up to 65% of postprandial insulin secretion. However, in subjects with type 2 diabetes, the incretin effect is diminished or no longer present.10 This is partially due to a reduction in the secretion of GLP-1 and a near complete loss of the effect of GIP in patients with type 2 diabetes.10,11 However, the effect of GLP-1 on insulin and glucagon is preserved in patients with diabetes, so that pharmacologic stimulation of GLP-1 receptors significantly reduces plasma glucose and improves glycemic control.3,7 Initial studies found that supraphysiological infusions of GIP in people with type 2 diabetes did not have an insulinotropic effect. However, current evidence suggests that GIP sensitivity can be restored in patients with type 2 diabetes through improved glycemic control.9,10
Incretin hormones have been implicated in energy intake and expenditure. GLP-1 promotes weight loss by delaying gastric emptying, increasing central satiety, and reducing food intake, leading to weight loss.3 Since GLP-1 secretion from the gut is impaired in obese patients, this may indicate a role for GLP-1 in the pathophysiology of obesity.3 Several GLP-1 receptor agonists are approved for the management of type 2 diabetes, including dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide. These agents have demonstrated a high level of glycemic efficacy while facilitating weight loss in patients with type 2 diabetes.
GIP receptors are widely expressed within the CNS and are found in areas implicated in regulating energy balance.9 Current evidence suggests that GIP has the potential to drive weight loss by directly targeting its receptor in the CNS to inhibit caloric intake or by enhancing the anorectic action of GLP-1.9 GIP also plays a role in dietary lipid storage. The GIP receptor is expressed in adipose tissue and enhances the ability of adipocytes to clear dietary triglycerides. By facilitating the expansion of white adipose tissue, GIP improves the long-term storage of lipids by adipocytes and reduces ectopic fat accumulation in tissues such as the liver, muscle, heart, and pancreas.9
The co-infusion of GIP and GLP-1 has a synergistic effect, resulting in enhanced insulin secretion and sensitivity, GIP-driven improvements in white adipose tissue function and lipid handling, and a strong anorexigenic effect from the activation of both receptor pathways in the brain.9,12 A dual GIP/GLP-1 receptor agonist is currently in phase 3 trials. Tirzepatide was developed by engineering GLP-1 activity into the sequence of the GIP peptide. Tirzepatide shows similar affinity for GIP receptors compared with native GIP but binds with the GLP-1 receptor with a 5-fold weaker affinity than native GLP-1. Binding with greater affinity to GIP than GLP-1 may provide an opportunity to take advantage of the synergy between the incretin hormones by fully engaging the GIP pathway while minimizing GLP-1-related tolerability issues.12 In a clinical trial of patients with type 2 diabetes, reductions in body weight were greater with 5 mg, 10 mg, and 15 mg of tirzepatide than with semaglutide (least-squares mean estimated treatment difference of -1.9 kg, -3.6 kg, and -5.5 kg, respectively; P< .001 for all comparisons). Tirzepatide doses of 5 mg, 10 mg, and 15 mg were also associated with a statistically significant reduction in A1c compared with semaglutide (estimated mean percentage change from baseline in A1c, -2.01, -2.24, -2.30 vs -1.86, respectively).13
References
- Lazzaroni E, Ben Nasr M, Loretelli C, et al. Anti-diabetic drugs and weight loss in patients with type 2 diabetes. Pharmacol Res. 2021;105782.
- American Diabetes Association. 8. Obesity Management for the Treatment of Type 2 Diabetes. Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S100-S110.
- Nauck MA, Meier JJ. Incretin hormones: Their role in health and disease. Diabetes Obes Metab. 2018;20(suppl 1):5-21.
- Hinnen D. Glucagon-like peptide 1 receptor agonists for type 2 diabetes. Diabetes Spectr. 2017;30:202-210.
- Meloni AR, DeYoung MB, Lowe C, Parkes DG. GLP-1 receptor activated insulin secretion from pancreatic β-cells: mechanism and glucose dependence. Diabetes Obes Metab. 2013;15:15-27.
- Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists. a review of their efficacy and tolerability. Diabetes Care. 2011;34(suppl 2):S279-S284.
- Holst JJ. The incretin system in healthy humans: The role of GIP and GLP-1.
2019;96:46-55. - Ramracheya R, Chapman C, Chibalina M, et al. GLP-1 suppresses glucagon secretion in human pancreatic alpha-cells by inhibition of P/Q-type Ca2+ Physiol Rep. 2018;6:e13852.
- Samms RJ, Coghlan MP, Sloop KW. How may GIP enhance the therapeutic efficacy of GLP-1? Trends Endocrinol Metab. 2020;31:410-421.
- Min T, Bain SC. The role of tirzepatide, dual GIP and GLP-1 receptor agonist, in the management of type 2 diabetes: The SURPASS clinical trials. Diabetes Ther. 2021;12:143-157.
- Lim GE, Brubaker PL. Glucagon-like peptide 1 secretion by the L-cell: The view from within. Diabetes. 2006;55(suppl 2):S70-S77.
- Willard FS, Douros JD, Gabe MBN, et al. Tirzepatide is an imbalanced and biased dual GIP and GLP-1 receptor agonist. JCI Insight. 2020;5:e140532.
- Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.



